Reviewing the Nomenclature for High-technology Trade – the Sectoral Approach
- Research
- Open Admission
- Published:
New indicators and indexes for benchmarking university–industry–authorities innovation in medical and life science clusters: results from the European FP7 Regions of Knowledge HealthTIES projection
Wellness Research Policy and Systems volume 17, Article number:10 (2019) Cite this article
Abstract
Background
While the European Union is striving to become the 'Innovation Spousal relationship', there remains a lack of quantifiable indicators to compare and criterion regional innovation clusters. To accost this outcome, a HealthTIES (Healthcare, Technology and Innovation for Economic Success) consortium was funded by the European union'due south Regions of Knowledge initiative, inquiry and innovation funding programme FP7. HealthTIES examined whether the wellness technology innovation cycle was functioning differently in five European regional innovation clusters and proposed regional and joint deportment to improve their performance. The clusters included BioCat (Barcelona, Catalonia, Spain), Medical Delta (Leiden, Rotterdam and Delft, South Holland, Netherlands), Oxford and Thames Valley (U.k.), Life Scientific discipline Zürich (Switzerland), and Innova Észak-Alföld (Debrecen, Republic of hungary).
Methods
Appreciation of the 'triple helix' of university–manufacture–regime innovation provided the impetus for the development of two quantifiable innovation indexes and related indicators. The HealthTIES H-index is calculated for disease and technology platforms based on the h-index proposed by Hirsch. The HealthTIES Innovation Alphabetize is calculated for regions based on 32 relevant quantitative and discriminative indicators grouped into 12 categories and three innovation phases, namely 'Input' (n = 12), 'Innovation System' (n = 9) and 'Output' (n = 11).
Results
The HealthTIES regions had developed relatively similar disease and technology platform profiles, still with distinctive strengths and weaknesses. The regional profiles of the innovation cycle in each of the 3 phases were surprisingly divergent. Comparative assessments based on the indicators and indexes helped identify and share best practice and inform regional and joint action plans to strengthen the competitiveness of the HealthTIES regions.
Determination
The HealthTIES indicators and indexes provide useful practical tools for the measurement and benchmarking of university–industry–government innovation in European medical and life science clusters. They are validated internally within the HealthTIES consortium and announced to have a caste of external prima facie validity. Potentially, the tools and accompanying analyses can be used beyond the HealthTIES consortium to inform other regional governments, researchers and, possibly, large companies searching for their next location, analyse and benchmark 'triple helix' dynamics within their own networks over time, and to develop integrated public–private and cantankerous-regional research and innovation strategies in Europe and across.
Background
Equally policy-makers meet innovation equally a fundamental driver of economic growth and wealth creation, the European Marriage (EU) is striving to become the 'Innovation Union' [1]. Despite substantial policy focus and investment in research and innovation at the regional level, comparing and benchmarking European regional innovation clusters is still in its formative stage and remains express in scope. Benchmarking commonly takes the form of observational studies of national innovation systems, sometimes with extended regional scoreboards. For example, the Regional Innovation Scoreboard was developed as an extension of the Innovation Union Scoreboard to measure the innovation performance of European regions on a express number of indicators [ii,iii,iv]. The Regional Ecosystem Scoreboard places emphasis on the dynamics and conditions that characterise the quality and nature of innovation and entrepreneurship in administrative regions, defined according to the Classification of Territorial Units for Statistics (NUTS) nomenclature of territorial units for statistical purposes [5]. The Cluster Observatory provides a range of macro- and micro-economical mapping data, reports on clusters and regional competitiveness conditions, information about cluster organisations, besides as educational resources [6]. In addition, a number of EU deputed reports highlight the varied quantification of the different phases of the innovation cycle, and the identification of critical factors that stimulate clustering from both a 'bottom-up' and 'top-down' perspective [7, 8]. The principal objective of such scorecards and reports are the relative ranking of countries and big administratively defined regions, but they practise not necessarily capture the workings and competitive advantages of actual innovation clusters from a regional co-ordination perspective [9, 10]. Moreover, information technology remains unlikely that they are sensitive to variations betwixt industry sectors, and therefore objective and quantifiable information tailored to the biotech, medical technology and pharma sectors are required for effective benchmarking and time dependent analysis [7, 11].
Herein, we focus on actual regional innovation clusters, defined as a critical mass of research innovation in academic institutions and companies that are located within a relatively small geographic area [12, 13]. Geographic clustering allows companies to derive competitive advantage, non just from their own resources and capabilities, only also from the shared resources and capabilities located in their geographical proximity [xiv]. Such geographic clustering is considered to encourage cantankerous-sector networks, spin-out companies, investment, collaborations and regional evolution, especially when in that location is a stiff presence of innovative firms from the aforementioned industry [15]. Interactions between large companies, small- to medium-sized enterprises and research organisations that pb to the sharing and exchange of facilities, knowledge and people, are probable to contribute to technology transfer. Importantly, these interactions may exist catalysed through extended networking, personnel and information dissemination within and between clusters [9]. Regions that have adult such clusters, especially those with a strong presence of universities and knowledge-based manufacture, are considered to accept enhanced economical competitiveness, innovation and growth [sixteen]. Given the benefits of clustering, national governments, the European Commission and intergovernmental organisations such as the OECD, advocate creating regional clusters, peculiarly in the areas that maximise public good [16,17,18,19,20,21]. The 'triple helix' of university–industry–government relations to increase the potential for innovation and wealth cosmos is now adult through new organisations such as science parks and incubators that cut across the academy–industry–government boundaries. Thus, quantitative benchmarking of regional clusters is an essential prerequisite for devising strategies and taking deportment to accelerate the impact of regional medical and life science clusters on health and wealth creation.
In the field of science, applied science and innovation (STI) studies, there are six established sources of data and related approaches [22] that can exist potentially used to quantify and measure out innovation with a high caste of comparability across a number of various regions. First, national-level research and development (R&D) data are used to characterise national contexts and inputs into the innovation process as well equally innovation activities [23]. Second, patents and citations provide insights into the invention procedure [24,25,26,27]. Third, bibliometrics help understand and forecast the scientific process underpinning inventions [28, 29]. Quaternary, technometrics rely on expert stance to assess technological change and its policy implications [30, 31]. Fifth, topic-specific databases and innovation surveys provide statistics on collaboration, commercialisation, financing and other innovation activities and opportunities [32,33,34]. Finally, blended synthetic indicators use a variety of information sources to assess innovation capabilities and operation [35, 36].
Edifice on the established STI approaches, nosotros report and discuss methods and results pertaining to the development of new HealthTIES (Healthcare, Engineering and Innovation for Economic Success) indicators and indexes for benchmarking academy–industry–government innovation in European medical and life scientific discipline clusters. The HealthTIES projection was part of the €126 one thousand thousand (2007–2013) Regions of Knowledge initiative supported by the European Commission under the inquiry and innovation funding programme FP7 [37]. The aim of the initiative was "to support trans-national mutual learning and cooperation betwixt enquiry-driven clusters, bringing together regional government and evolution agencies, public enquiry organisations, industry and other relevant stakeholders" [37]. The main activities adult by HealthTIES to achieve the aims of Regions of Noesis were to complete a detailed gear up of quantitative assessments for the analysis of regional clusters, including the development of a 'virtual reference cluster', to develop a mentoring strategy between a highly developed cluster (Life Science Zürich) with one less developed (Debrecen, Hungary), and to develop a set of regional action plans to improve the integration of contributors to regional economies.
Given that the primary focus of our project was on the HealthTIES regions, nosotros initially disseminated and used the results of the project to inform actions within the regions. Nevertheless, there are a paucity of such research outputs from this and other projects in the public domain and peer-reviewed literature [38], similar to what is observed for other European-funded projects and initiatives. A recent written report in Wellness Research Policy and Systems demonstrated that, although significant public resource are invested in European-funded health projects, data and reports from such projects are frequently unavailable or inaccessible [39]. Globally, billions of dollars in research investment are wasted when full information about wellness research studies is inaccessible [40]. In line with the objectives of Responsible Research and Innovation [41], which increases the value of European research investment, we herein make the results from the HealthTIES project publicly available in Open Access format.
Methods
Aim and objectives
Nosotros established the HealthTIES consortium [42] to deliver on the stated aim of the Regions of Knowledge by examining whether the health technology innovation cycle was functioning differently in v European regional innovation clusters, and to propose regional and joint actions to improve their performance. The specific objectives were every bit follows:
-
develop indicators and indexes for assessing knowledge and innovation capital and the functioning of the innovation cycle;
-
identify key stakeholders and collect information to operationalise the proposed indicators and indexes;
-
propose articulation and regional actions to improve regional performance through benchmarking and mutual learning.
Study setting
The HealthTIES consortium consisted of five European regional clusters centred around the following cities (Fig. ane):
-
Barcelona (Bioregion of Catalonia, Catalonia),
-
Leiden, Rotterdam, and Delft (Medical Delta, Due south Holland),
-
Oxford (Oxford and Thames Valley, Uk),
-
Zürich (Life Science Zürich, Switzerland),
-
Debrecen (Innova Észak-Alföld, Hungary).
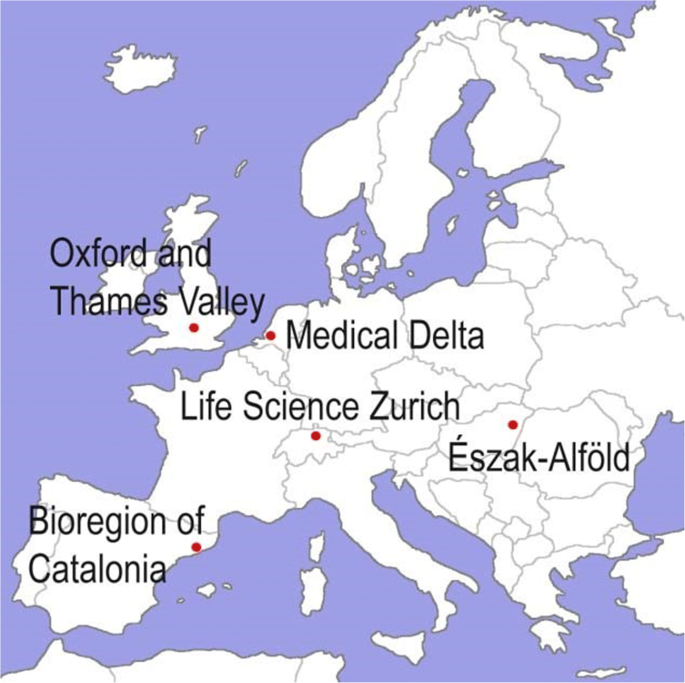
The HealthTIES (Healthcare, Technology and Innovation for Economic Success) consortium
We selected the first four participating clusters on the ground of pre-existing bilateral collaboration between cluster members and their proven track tape in medical and life sciences research and innovation [38]. Innova Észak-Alföld was selected equally a mentoring cluster on the basis of its potential to accelerate inquiry-driven innovation. Hungary has one of the most developed biotechnology sectors among the new EU fellow member states, and Debrecen had attracted significant investments from Gedeon Richter Plc. to build a new biotechnology product facility that is unique in Central Eastern Europe [43].
Theoretical framework
To guide data collection, analysis and actions, this study used the established 'triple helix' model of academy–manufacture–government relations based on the theoretical insight that universities, industry and authorities are becoming increasingly interdependent and co-evolving, while retaining their institutional identities [17, 21, 44,45,46]. Imperative to the functioning of the 'triple helix' is the creation of the 'noesis space' to intensify research and generate new knowledge through collaboration, the 'consensus space' to build relationships, agree on objectives and develop joint actions, and the 'innovation infinite' to implement joint actions past bringing together knowledge, business organization expertise and venture capital. The 'new organisational actors' or 'hybrid organisations', such as science parks, incubators and new forms of venture majuscule, which span traditional university–industry–regime boundaries, are shown to be effective in creating the aforementioned 'spaces' [21, 47, 48].
Under the assumptions of 'triple helix' model, maximising interactions, creating 'spaces' and new organisations that cutting beyond the university–industry–government boundaries are proposed to increase the potential for innovation and wealth creation through costless motility of people, knowledge and venture capital between universities, manufacture and authorities.
Information drove and assay
For each cluster, nosotros nerveless and analysed the nigh recent data pertaining to universities, manufacture, government and new boundary-spanning organisations during the lifespan of the projection (2010–2013). Most of our cantankerous-sectional data and analyses refer to 2010 or 2012. We used a variety of well-established STI sources of data and approaches to information analysis. Namely, we collected and analysed R&D data from statistical offices and institutions, bibliometric data, public and commercial databases, and a number of readily bachelor composite synthetic indicators. These data and analyses formed the basis of the HealthTIES H-index for disease and technology platforms and the HealthTIES Innovation index focusing on four affliction fields (cardiovascular disease, cancer, neurodegenerative disease, immunology and infectious disease) and iii engineering platforms (molecular technology, imaging, and drug pattern, evolution and delivery).
We selected these affliction fields and applied science platforms on the basis of the concurrent assessment of the burden of mortality and morbidity in Europe also as scientific and technological alter and by the project'southward experts equally the most promising for improving the health of European citizens and making European health systems more sustainable in the context of the increasingly ageing population. Cardiovascular illness, cancer, infectious diseases and neurodegenerative diseases are the leading causes of mortality and morbidity in Europe. Oftentimes, they are manifestations of patient/gene–environment interactions. Therefore, interventions directed at environmental risk factors, such as alcohol, obesity and tobacco, are likely to have of import simply mixed benefits depending upon underlying genetic factors and age at exposure. Recent scientific and technological breakthroughs in molecular applied science and imaging, likewise as drug design, development and delivery have opened upwardly new opportunities for primary prevention of these diseases through gamble factor identification and secondary prevention through early and accurate diagnosis, too as more appropriate and constructive treatments.
HealthTIES H-index for affliction and engineering science platforms
To assess the cognition and innovation uppercase in each region we used bibliometric h-indexes [49]. Although most unremarkably h-indexes are calculated for individual researchers, they could also be calculated for regions, diseases and technologies. Nosotros calculated h-indexes using the Clarivate Web of Scientific discipline, formerly Web of Knowledge, enquiry platform (www.webofknowledge.com) to benchmark our regions in four relevant illness fields and 3 technology platforms. Our protocol and search terms are shown in Additional file 1: Box S1 and Table S1, respectively. The HealthTIES h-index (HT H-index) for publications in a region in a specific field specifies the number HT of scientific manufactures for a given keyword (i.e. cardiovascular) for a given address (eastward.g. Zürich) that have been cited in other articles at to the lowest degree HT times according to Hirsch [49]. As the h-index depends on the length of a person'southward scientific career, the HT H-index depends on the volume of research activeness within clusters. The HT H-index permits the comparison of the relative strengths of a given location for specific keyword search terms and thus offers a valuable assessment of the research output of a cluster in a given disease or engineering science platform.
HealthTIES innovation index
In an effort to create a 'consensus space' for edifice relationships, agreeing on objectives and developing articulation actions under the assumptions of the 'triple helix' model, each cluster assembled a regional team, including representatives from small- to medium-sized enterprises, biotech parks, local government, hospitals, technology transfer offices (TTOs) and academia. Initially, nosotros collected a broad range of data for each region, but it became clear that these data were either not necessarily bachelor for each region, or were not comparable, or were not quantifiable. A workshop was convened to talk over the all-time data to collect and how to employ this to an alphabetize. All regional clusters were represented by 15 members, extending beyond every strand of the 'triple helix'. We had discussions near the significance, validity, reliability and feasibility of the potential indicators facilitated by an independent consultant, and we reached consensus on which indicators to select. As a result, we adult a logic model of the innovation cycle consisting of 3 fundamental phases, namely input, innovation system activity and outputs (Fig. 2).
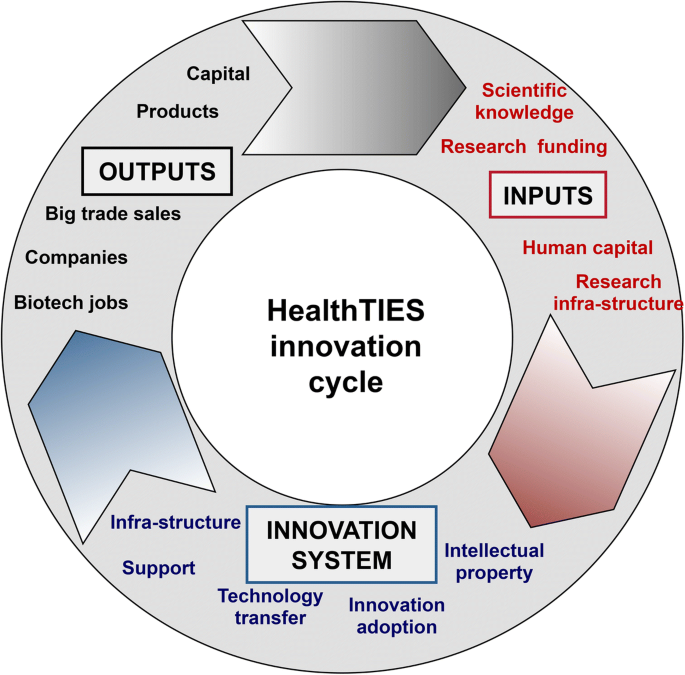
A logic model of the HealthTIES (Healthcare, Technology and Innovation for Economic Success) innovation bike with key indicator categories
Nosotros then compiled a list of potential indicators for each phase of the innovation cycle excluding those indicators for which no data was available or would not be feasible to collect. This process resulted in 32 quantitative indicators, which were grouped into 12 categories and three phases of the innovation cycle, namely 'Input' (due north = 12), 'Innovation Arrangement' (n = 9) and 'Output' (n = 11) (Table 1). We too assigned, to each indicator, a weighting cistron reflecting the relative significance of the underlying component in the innovation cycle. We adamant mean scores for each weighting gene by voting amidst the 15 members present at the meeting, followed by wider apportionment to all the members of the regional steering/advisory committees requesting their feedback, amendments and endorsement of the last set of indicators and weighting factors (Table 1).
Input indicators
The concluding set includes 12 indicators to measure inputs in iv categories. First, the numbers of highly cited professors and publications were accounted to be proxy indicators for the depth and breadth of the current scientific cognition base of operations. Given that the h-index reflects the duration of 1's scientific career, an h-index > thirty was called as a threshold for highly cited professors to likewise include younger researchers who had already made a significant impact on their respective fields. 2nd, the scope of the current research activity was measured through research funding. In doing then, currencies other than the euro were converted into euros using historical exchange rates. 3rd, both quantity and quality of human upper-case letter were considered to be key to current and future discoveries and innovation. Nosotros measured the numbers of graduated national and international MSc and PhD students as well equally the numbers of European Inquiry Council junior and advanced grants, which serve equally a pan-European mark of inquiry quality. 4th, to guess research infrastructure for current and hereafter research, we measured research infinite, research hospital beds, and Phase I and Two clinical trials. The latter indicate activeness levels in proof of concept drug development.
Innovation system indicators
There are nine indicators to measure out innovation arrangement activity in 3 categories. Kickoff, the existing levels of innovation that were deemed to be reflected in intellectual holding, university–industry applied science transfer and adoption of innovation. Intellectual holding and academy–manufacture engineering science transfer were measured by the numbers of university spin-off companies, granted United States patents, and the number of academy–manufacture collaborations larger than 5 meg euros. The patients Expect indicator (patients Waiting for Access to Innovative Treatment) was used to measure how fast innovations and new drugs are made available to the public [50]. Second, support for innovation was measured by the number of full-time equivalent (FTEs) posts in academy TTOs in a given region and the extent of national regime procurement of advanced applied science. Tertiary, the quality of innovation infrastructure was accounted to be reflected within the regional science park capacity and the overall national attractiveness. The capacity of regional science parks was measured by the size of infinite available in square meters and past the number of support staff in FTEs. The national bewitchery was deemed to be reflected past the Earth Economical Forum Global Competitiveness alphabetize [51].
Output indicators
There were 11 indicators to measure direct and more firsthand outputs of the innovation system in 5 categories. First, the number of jobs in biotech companies was deemed to be i of the cardinal indicators for economical growth and wealth creation. Second, nosotros measured the number of non-subsidiary independent biotech companies, distinguishing between smaller companies (with fewer than 20 FTE employees) and larger companies (with 20 or more than employees). Third, we measured the number of big trade sales (larger than 100 1000000 euros), where small companies financed by venture upper-case letter or private equity were bought up by big life science, medical technology, biotech or pharma companies. Fourth, we measured the number of regional biotech companies' products and the full number of medicines available nationally. The number of regional biotech companies' products included those on the market, in clinical trials phases I–3 and pending Usa Nutrient and Drug Administration approval, and in the pipeline, including discovery and lead optimisation. Finally, we measured capital as reflected in the full amount of investments (in euros), number of investments and the value of average Series A investments, i.e. the first pregnant venture upper-case letter investments. Given that there was no common publicly available data source for all of the HealthTIES regions, nosotros operationalised products and capital indicators using the BiotechGate database (www.biotechgate.com) established by Venture Valuation Ltd., located in Zürich. BiotechGate is the largest life science visitor database in Europe and the United States, established primarily for the purposes of supporting partnering meetings. They allowed u.s. to search our specific regions in their BiotechGate database (www.biotechgate.com). Although information technology is the virtually comprehensive database that is currently available, it is not exhaustive and is dependent on voluntary registration. Therefore, comparison of the indicators derived from this database may take known limitations.
Comparison and benchmarking
All indicator data were converted into points, on linear scales ranging from 0 to 10, with ten being the highest aspirational score. The highest actual information value of an indicator in any HealthTIES region was set at 7.v points. This implied that each region may improve to 10 points in the futurity. This presentation method was applied to both HT Innovation and H-indexes. Equally stated, each HT Innovation indicator was so attributed by the HealthTIES consortium consensus, a relative weighting factor (Table ane). Overall scores were calculated by multiplying indicator points by the corresponding weighting factors. Category scores were obtained past adding up indicator scores within a category. Radar plots were used to visualise and compare indicator and category scores. The original benchmarking results for 2010 and 2012 are reported beneath and in Boosted file 1.
Results
Comparative assessment of the knowledge and innovation majuscule
Choice of mutual bibliometric indicators enabled the comparing of the noesis and innovation capital in regional clusters across operationally independent disease and technology platforms (Fig. three). Strikingly, all four avant-garde regions had developed a relatively like shape of illness and engineering platform profiles, still with distinctive strengths and weaknesses. For example, the forcefulness of microscopy in Zürich and its weakness in Barcelona (Bioregion of Catalonia; BioCat), or the forcefulness of clinical trials in Oxford and Medical Delta and their weakness in Zürich.
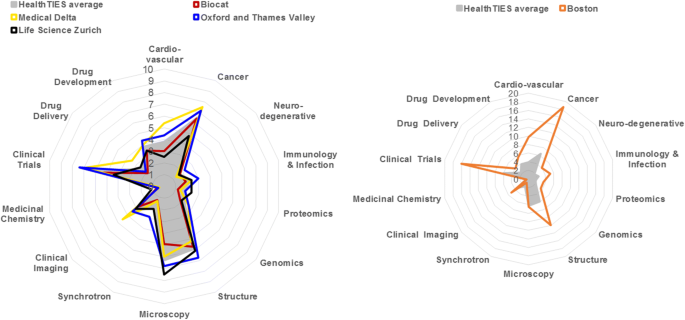
A comparative cess of the knowledge and innovation capital using the HealthTIES H-index for disease and technology platforms, 2010. Data are expressed relative to a maximum of 7.5 in the all-time performing HealthTIES region
We also compared the average HealthTIES affliction and engineering science platform profile with the profile of Boston, which is a renowned metropolitan surface area in life science innovation [52]. Nosotros found that the shape of disease and technology platform profiles was similar, but of significantly greater magnitude in Boston (Fig. 3). The relative similarity of regional profiles across Europe, especially with respect to cancer and cardiovascular disease, is likely to reflect the relatively like priorities for healthcare funders. By contrast, the relatively limited activeness in the public domain in chemistry and drug development is likely to reflect the concentration of this activity in the pharmaceutical industry.
Comparative assessment of the innovation wheel using the HealthTIES Innovation Index
The profiles obtained for each regional cluster in each of the iii phases of the innovation ecosystem were surprisingly divergent (Fig. 4 and Boosted file 1: Figure S1–S5). Thus, with a unmarried time point or snapshot, information technology was difficult to decide the exact cause and effect relationships between indicators. This could also propose that no one uniform template for regional innovation clusters exists in Europe, and that variation may be regional and context dependent.
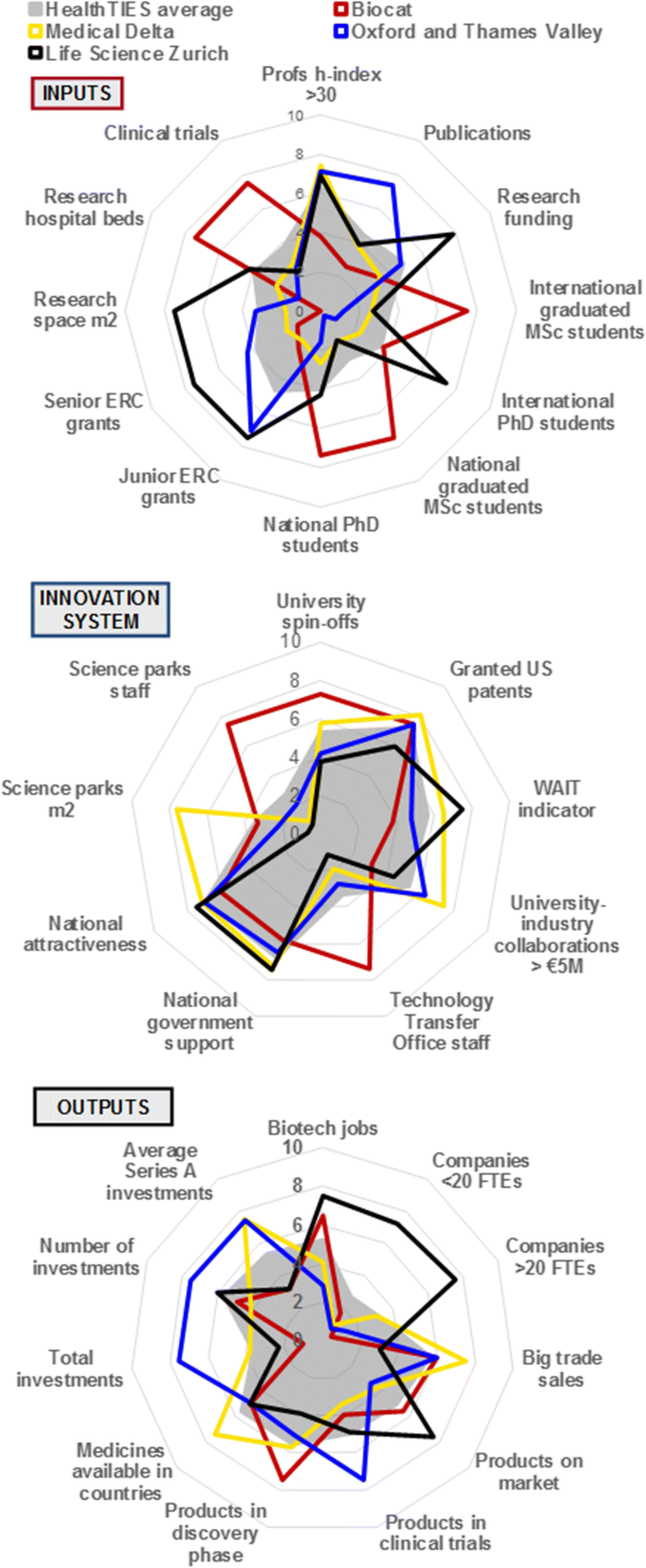
A comparative assessment of the innovation wheel using the HealthTIES Innovation index, 2010
In support of the utility of the HT Innovation index over time, the changes in data over two periods was determined in 2 regions where we could reassess all indicators (Additional file 1: Effigy S6). These data strongly suggest that there is a time-dependent dynamic of the investment, innovation and outputs along the innovation bicycle, illustrating the need for temporal assessments to evaluate fluctuations and (in)stability of the cluster. Over a given time, Life Science Zürich developed both investment and products on the market, whereas Oxford and Thames Valley and Medical Delta have achieved major increases in investment, just with little change in product or outputs in this menstruum. Yet, Oxford and Thames Valley accept a number of products in development, and this probably reflects an accent on clinical trials. Without a quantifiable HT Innovation index with temporal evaluation, there is a risk that these information could exist misinterpreted. For instance, the effect of increasing external investment in one particular expanse may not take impact on wealth cosmos. Likewise, increasing forrad intellectual holding without correlated evolution of investment may not translate to potential impacts on healthcare outcomes.
HealthTIES indexes inform regional action
The indexes were so used to identify the relative strengths, weaknesses and opportunities in each cluster to benchmark regions, and provide a fix of mutual innovation indicators that could be readily applied to any comparable cluster over time. For the gross differences between regions, the strengths, weaknesses, opportunities and threats (SWOT) and gap analyses were internally validated within the HealthTIES consortium. Moreover, equally medical and life sciences represent a sizable part of the enquiry and innovation organisation in each of the HealthTIES regions, comparisons of our findings for medical and life science clusters in the HealthTIES regions with the rankings of their respective countries and administrative regions beyond all sectors announced to signal a degree of external prima facie validity of the HealthTIES indicators and indexes. In line with our findings that Life Scientific discipline Zürich outperforms other HealthTIES regions on the greatest number of indicators, the European Innovation Scoreboard shows that Switzerland is the overall innovation leader in Europe, outperforming all Eu member states [iv]. Similar to the Regional Innovation Scoreboard (RIS) [5] ranking, the Oxford and Thames Valley region was a loftier innovator and BioCat and Medical Delta (only partial RIS information) were medium-loftier innovators. The HealthTIES indexes indicate that Oxford and Thames Valley performs slightly meliorate than BioCat and Medical Delta. Both the RIS and HealthTIES study revealed that Debrecen is a low innovator.
Importantly, some of the differences betwixt medical and life scientific discipline clusters in the HealthTIES regions could be addressed in the local regions (Additional file 1: Effigy S7). In an effort to create the 'innovation space' under the assumptions of the 'triple helix' model, the initial regional and joint actions following the adoption of the HealthTIES Innovation Indicators included the installation of formal 'triple helix' organisations (consisting of academia, manufacture and government), professionalisation of the academic TTOs, investing in entrepreneurship educational activity, combined with incubator facilities and international entrepreneurs in residence programmes (Box 1). A set of joint actions could also atomic number 82 to coupling and collaboration of some of Europe'southward almost innovative biotech regions such as combining business development between regions, public–private R&D and entrepreneurship education. Developing a more detailed regional roadmap for further actions would require a more than integrated approach with 'triple helix' actors, comprehensive time-dependent information and prospective evaluation following each initiative.
Best practice in bioincubation
Identifying and sharing best practice in bioincubation provides an illustrative case of regional actions following the adoption of the HealthTIES innovation indicators. A bioincubator is a concrete environment with labs and offices where companies tin can start up, but also have admission to experienced entrepreneurs, venture capital vehicles, skills grooming, mentoring and business services located in shut proximity to major universities, research institutions, hospitals and life science companies. Almost often, bioincubators are new 'triple helix' organisational actors that cut across the university–industry–authorities boundaries. In 3 HealthTIES regions (BioCat, Medical Delta and Life Scientific discipline Zürich), bioincubators proved to exist successful in stimulating innovation and economical growth by creating new high-tech companies and jobs, and therefore the Oxford regional team carried out site visits and in-depth interviews to study all-time practice in setting up and running bioincubators with a view to developing a bioincubator for Oxford and Thames Valley.
Major challenges in learning from international best exercise concerned the transferability of findings to the intended setting. First, the functioning of bioincubators appeared to be dependent on laws, taxes and other country-specific institutions. Therefore, to increase the transferability of best practice to the U.k. context, in add-on to 5 bioincubators in HealthTIES regions, we studied a farther 6 bioincubators in England and Scotland. 2d, the location and proximity of a bioincubator to major universities, research institutions, hospitals and life scientific discipline companies emerged as the nearly frequently cited forcefulness of the bioincubators studied. To capitalise on this forcefulness, we studied in-depth BioPartner Centre Leiden, which emulates the proximity of the university and hospitals in Oxford better than any other bioincubator studied. Tertiary, funding and sustainability in the first ten years of functioning emerged as the most oft cited weakness of the bioincubators studied. At that place was a high degree of variation in how different bioincubators raised the investment necessary to build and equip the bodily physical environment, attracted start-up companies, and generated revenue from multiple public and individual sources over time. As no uniform business model emerged, we summarised a diverse range of all-time exercise in setting upwardly and running bioincubators in a concern model sail (Additional file 2).
Discussion
This report reports the results and outcomes from the European FP7 Regions of Knowledge HealthTIES project. Specifically, our study provides tools for the measurement and benchmarking of university–industry–government innovation in selected European medical and life science clusters. The general outcome is that the HealthTIES indicators and indexes complement the existing generic tools for measuring innovation operation across all sectors at the level of countries and large administratively divers regions by focussing on actual innovation clusters in ane specific sector. The very nature of this comparative process means that many indicators that are potentially informative were subsequently not used because of the lack of universal availability. At that place are therefore limitations to all such tools and indicators that are based on what can be measured, rather than on what should be measured. With respect to these caveats, the HealthTIES indicators and indexes accept value, as they been co-developed and validated internally inside the HealthTIES consortium of regional partners with representation from academia, government and industry, and appear to have a degree of external prima facie validity.
In add-on, the HealthTIES indicators and indexes provided aplenty information for SWOT analysis, which in plow formed the footing of our regional and joint action plans. Our analysis shows that, although HealthTIES innovation regions possess a strong knowledge base of operations and innovation infrastructure in the four mature clusters, and a stiff potential for development in the mentoring cluster, they all significantly lag behind the world-leading Boston cluster (Fig. 3). This is a reflection of Europe being outperformed by the global innovation leaders such equally South Korea, Canada, Commonwealth of australia, Nihon and the U.s.a. [4]. Although the European union still has a clear innovation performance lead over many other countries globally, this atomic number 82 is declining rapidly and comes nether threat from countries such as China, whose innovation functioning growth is three times that of the European union [iv].
HealthTIES had regional value, as we used the results from the comparative cess of the innovation cycle to identify and share best practice and inform regional and articulation activeness plans to increase the competitiveness of the HealthTIES regions. There was also a synergistic effect from involving regional stakeholders across academia, manufacture and government in gathering and interpreting data. The HealthTIES projection likewise raised sensation and increased commitment to alter amongst the traditional 'triple helix' partners (academia, government, and medical and life science industry), new partners such equally insurance companies and individual investors, and the general public.
With regard to the particular areas of the cognition and innovation capital letter, our written report shows a major deficiency in the area of medical chemistry across all five regions. The lack of critical mass in this area in academia and publicly funded inquiry institutes may exist compensated by a larger volume of research in the pharmaceutical industry. All of the regions announced to exist dependent on a steady drug development pipeline in the pharmaceutical industry, including vaccines and biological agents. In view of the changes that are ongoing in the pharmaceutical industry, these dependencies present a potential threat to the connected high-level action in the HealthTIES clusters. For example, a major fall in revenue for pharma is expected equally the patent lifetime of many drugs is coming to an end and they approach what has been termed the 'patent cliff' [53]. This fall in revenue and the increasing costs of getting drugs through Phase III, United States Food and Drug Administration and European Medicines Agency blessing, mean that many drug development programmes are folding [54]. To counteract this threat in the context of P4 (predictive, preventive, personalised, participatory) medicine, academia and government across Europe may demand to play a more agile role in basic research and drug development in partnership with industry.
Limitations and hereafter enquiry
The HealthTIES indicators and indexes have a number of limitations. Starting time, our report was based on the assumptions of the 'triple helix' model to guide information collection, analysis and actions. Testing the underlying assumptions was beyond the scope of the study, every bit the 'triple helix' was regarded as a starting point. As a consequence, our assay and proposed actions focused on the dynamic interactions inside the 'triple helix' to generate a pragmatic, measurable and effective evaluation of the parameters influencing the innovation cycle. It is probable that this approach volition have focused activity at the expense of other sectors. For instance, taking into the account the credible dependency of the HealthTIES regions on research and drug development in the pharmaceutical manufacture, incentivising R&D investment in the pharmaceutical manufacture through tax breaks could be either more than or equally advantageous to some of the deportment we considered. Nosotros intend to echo data collection and HealthTIES innovation indicator assay after implementation of the initial regional and joint actions. The side by side steps in evaluating the utility of the HealthTIES indicators and subsequent deportment should include wider evaluation of the outcomes of the actions based on the assumptions of the 'triple helix' model, as well every bit comparison of their advantages and disadvantages with a range of possible alternative actions to accelerate innovation.
Second, quantifying and measuring innovation is inherently challenging considering, by definition, information technology is something novel and the settings within which it occurs are multidimensional and in many ways unique. To ensure comparability and quantitative measurability, our approach uses a variety of indirect innovation indicators (e.g. inquiry funding, publications, patents and investments) too as quantitative direct innovation indicators (east.g. new products on the market, new medicines, big sales). An inherent limitation of such an arroyo is that it does not focus on the qualitative characteristics of the object of innovation, i.e. innovative products, services and technologies. Future research may explore alternative approaches focusing directly on the object of innovation to investigate whether the new products, services and technologies are incremental improvements or disruptive innovations, whether they have a potential to become blockbuster drugs or high-quality services, and whether they have occurred within or exterior of the formal medical and life science R&D sector.
Third, within the inherent limitations of the current arroyo, in that location are considerable challenges in obtaining consummate, reliable and comparable datasets, particularly for innovation system and output indicators. We were unable to include in our analysis a number of indicators, data for which were not available for all regions, or were non comparable or quantifiable. Particularly, there may be limitations to the reliability of some of the information collected for the HealthTIES regions. For case, companies consummate their ain entries in the BiotechGate database, and so their entries may vary in terms of quality, consistency and reporting periods. Moreover, the electric current version of the innovation indicators is based on absolute rather than relative measurements and thus does not take into consideration differences among HealthTIES regions with regards to the size and quality of various sociodemographic characteristics. Future enquiry may usefully develop a methodology to business relationship for such differences and to collect more complete, reliable and comparable data, for example, through comprehensive and cantankerous-validated innovation surveys.
Fourth, external validation of the HealthTIES indicators and indexes was beyond the telescopic of the current projection. Futurity research may usefully validate them in other settings using both quantitative and qualitative validation techniques. In detail, at that place may exist a problem in using some of the readily available indicators across different settings. For example, the patients Wait indicator typically includes the innovations and new drugs that have undergone marketplace authorisation just are all the same to undergo Phase 4 clinical trials to make up one's mind their long-term effects on large populations, besides as health technology assessment to determine their price effectiveness. Thus, some innovations and new drugs included in this indicator in some settings may accept agin long-term furnishings or may non be price-effective. Our feel of conducting a workshop with a wide range of experts including, amidst others, front-line clinicians to build a consensus on the choice and feasibility of innovation indicators suggest that member validation and consensus research are likely to be effective ways to gather expert opinion and build a consensus on the utility and feasibility of different indicators in the context of different regions.
Finally, our study did not explore qualitative differences between the clusters with regard to the workforce, business practices and laws, and how they support or forestall innovation. Yet, our previous research in i of the HealthTIES regions shows that innovation, entrepreneurship and collaboration culture varies between dissimilar healthcare and research organisations and may affect both health and economic outcomes [20, 55, 56]. Moreover, women remain under-represented in leadership and management positions in European academic health centres [57], which indicates that research systems may non support the best scientific discipline or fail to address topics that benefit women and men deservedly [58]. Futurity research may usefully explore qualitative differences between organisations, sectors and regions using theory-building research designs to draw generalisations beyond the current sample of medical and life science clusters.
Decision
Of import sectors of the economic system, such as medical and life sciences, increasingly revolve around a 'triple helix' of university–industry–government relations at both national and regional levels. In lite of growing interdependencies between previously arms-length actors, it is vital for research and innovation stakeholders and policy-makers to have access to tools for measuring, monitoring and comparing 'triple helix' dynamics in key sectors. The HealthTIES indicators and indexes provide useful practical tools for the measurement and benchmarking of university–industry–government innovation in European medical and life science clusters. They have been developed and validated internally within the HealthTIES consortium and display a degree of external prima facie validity. The results from the comparative cess of the innovation cycle have been used to identify and share best practice and inform regional and joint action plans to strengthen the competitiveness of the HealthTIES regions. Potentially, the tools and accompanying analyses can be used across the HealthTIES consortium to inform other regional governments, researchers and, perhaps, large companies searching for their next location, analyse and benchmark 'triple helix' dynamics within their own networks over time, and to develop integrated public–private and cantankerous-regional research and innovation strategies in Europe and across.
Abbreviations
- BioCat:
-
Bioregion of Catalonia
- ERC:
-
European Enquiry Council
- European union:
-
European Matrimony
- FTE:
-
full-fourth dimension equivalent
- HealthTIES:
-
Healthcare, Engineering science and Innovation for Economical Success
- HT H-index:
-
HealthTIES h-index
- R&D:
-
research and development
- RIS:
-
Regional Innovation Scoreboard
- STI:
-
science, engineering and innovation
- SWOT:
-
strengths, weaknesses, opportunities and threats
- TTO:
-
engineering science transfer office
- WAIT:
-
patients Waiting for Access to Innovative Treatment
References
-
European Commission. Innovation Matrimony: A Europe 2020 Initiative. http://ec.europa.eu/research/innovation-union/index_en.cfm. Accessed 20 Jun 2018.
-
Schibany A, Streicher Yard. The European Innovation Scoreboard: drowning by numbers? Sci Public Policy. 2008;35(10):717–32.
-
Foray D, Hollanders H. An assessment of the Innovation Marriage Scoreboard every bit a tool to analyse national innovation capacities: the case of Switzerland. Res Eval. 2015;24(2):213–28.
-
European Commission. European Innovation Scoreboard. http://ec.europa.eu/growth/industry/innovation/facts-figures/scoreboards_en. Accessed twenty Jun 2018.
-
European Commission. Regional Innovation Scoreboard. http://ec.europa.eu/growth/manufacture/innovation/facts-figures/regional_en. Accessed xx Jun 2018.
-
Center for Strategy and Competitiveness. Cluster Observatory. http://www.clusterobservatory.european union/. Accessed i Mar 2017.
-
European Committee. Regional Biotechnology: Establishing a Methodology and Operation Indicators for Assessing Bioclusters and Bioregions Relevant to the KBBE Expanse. https://www.pwc.de/de/offentliche-unternehmen/assets/neues-denken/regional-biotech-report.pdf. Accessed 20 Jun 2018.
-
Arundel A, Casali L, Hollanders H. How European public sector agencies introduce: the apply of lesser-up, policy-dependent and noesis-scanning innovation methods. Res Policy. 2015;44(7):1271–82.
-
European Committee. Framework for Land Aid for Research and Development and Innovation. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52014XC0627%2801%29. Accessed 20 Jun 2018.
-
Saxenian A. Lessons from Silicon Valley. Technol Rev. 1994;97(5):42–51.
-
Malerba F. Sectoral systems of innovation and production. Res Policy. 2002;31(2):247–64.
-
Porter ME. Clusters and the new economics of competition. Harv Passenger vehicle Rev. 1998;76(6):77–90.
-
Porter ME. Location, contest, and economic development: local clusters in a global economic system. Econ Dev Q. 2000;14(i):15–34.
-
Davis CH, Arthurs D, Cassidy E, Wolfe D. What indicators for cluster policies in the 21st century? www.oecd.org/sti/inno/37443546.pdf. Accessed 1 Mar 2017.
-
Beaudry C, Breschi S. Are firms in clusters actually more innovative? Econ Innov New Technol. 2003;12(four):325–42.
-
Remoe South, Guinet J. Dynamising National Innovation Systems. Paris: OECD; 2002.
-
Etzkowitz H, Leydesdorff LA. Universities and the Global Noesis Economic system: A Triple Helix of Academy-Industry-Government Relations. London, New York: Pinter; 1997.
-
Potter J, Miranda G. Clusters, Innovation and Entrepreneurship. Paris: OECD; 2009.
-
European Commission. Capacities Work Programme: Regions of Knowledge. http://ec.europa.eu/inquiry/participants/data/ref/fp7/132062/q-wp-201301_en.pdf. Accessed xx Jun 2018.
-
Ovseiko PV, O'Sullivan C, Powell SC, Davies SM, Buchan AM. Implementation of collaborative governance in cantankerous-sector innovation and pedagogy networks: evidence from the National Wellness Service in England. BMC Health Serv Res. 2014;xiv:552.
-
Etzkowitz H. The Triple Helix: University-Industry-Authorities Innovation in Action. Abingdon-on-Thames: Routledge; 2008.
-
Smith K. Measuring innovation. In: Fagerberg J, Mowery DC, editors. The Oxford Handbook of Innovation. Oxford: Oxford University Press; 2006. p. 1–38.
-
OECD. Frascati Transmission: Guidelines for Collecting and Reporting Data on Research and Experimental Development. http://oe.cd/frascati. Accessed 12 Dec 2018.
-
Acs ZJ, Anselin L, Varga A. Patents and innovation counts every bit measures of regional production of new knowledge. Res Policy. 2002;31(7):1069–85.
-
Crosby One thousand. Patents, innovation and growth. Econ Rec. 2000;76(234):255–62.
-
Callaert J, Van Looy B, Verbeek A, Debackere Yard, Thijs B. Traces of prior art: an analysis of non-patent references found in patent documents. Scientometrics. 2006;69(i):three–twenty.
-
Ovseiko PV, Oancea A, Buchan AM. Assessing enquiry bear upon in academic clinical medicine: a study using Inquiry Excellence Framework pilot affect indicators. BMC Health Serv Res. 2012;12:478.
-
Teixeira AAC. Evolution, roots and influence of the literature on National Systems of Innovation: a bibliometric business relationship. Camb J Econ. 2014;38(1):181–214.
-
Watts RJ, Porter AL, Newman NC. Innovation forecasting using bibliometrics. Competitive Intell Rev. 1998;9(4):11–nine.
-
Grupp H. The measurement of technical performance of innovations by technometrics and its touch on established technology indicators. Res Policy. 1994;23(ii):175–93.
-
Coccia M. Technometrics: origins, historical evolution and new directions. Technol Forecast Soc Chang. 2005;72(eight):944–79.
-
Eurostat. Community Innovation Survey (CIS). https://ec.europa.eu/eurostat/web/microdata/community-innovation-survey. Accessed 12 Dec 2018.
-
BiotechGate. Concern development database for the life science industry. https://www.biotechgate.com. Accessed 12 December 2018.
-
OECD. Eurostat Oslo Manual 2018; 2018. http://www.oecd.org/sti/inno/oslo-manual-2018-info.pdf. Accessed 21 January 2019.
-
Archibugi D, Denni Yard, Filippetti A. The technological capabilities of nations: The state of the art of synthetic indicators. Technol Forecast Soc Chang. 2009;76(vii):917–31.
-
Grupp H, Schubert T. Review and new evidence on blended innovation indicators for evaluating national performance. Res Policy. 2010;39(1):67–78.
-
European Commission. Regions of Knowledge. http://ec.europa.european union/invest-in-research/funding/funding03_en.htm. Accessed twenty Jun 2018.
-
Lawton Smith H, Bagchi-Sen Due south, Edmunds L. Innovation cycles and geographies of innovation: a report of healthcare innovation in Europe. Eur Urban Reg Stud. 2017;25(4):405–22.
-
Zander B, Busse R. Is at that place enough research output of EU projects available to assess and improve wellness system performance? An effort to understand and categorise the output of European union projects conducted between 2002 and 2012. Wellness Res Policy Syst. 2017;xv:13.
-
Chan AW, Song F, Vickers A, Jefferson T, Dickersin Chiliad, Gotzsche PC, et al. Increasing value and reducing waste matter: addressing inaccessible research. Lancet. 2014;383(9913):257–66.
-
2014 Italian Presidency of the Council of the European Union. Rome Proclamation on Responsible Inquiry and Innovation in Europe. https://ec.europa.eu/inquiry/swafs/pdf/rome_declaration_RRI_final_21_November.pdf. Accessed 14 December 2018.
-
CORDIS. Health-TIES. https://cordis.europa.eu/projection/rcn/95846_en.html. Accessed twenty Jun 2018.
-
EuropaBio, Venture Valuation. Biotech in the New Eu Fellow member States: An Emerging Sector 2009. http://www.gate2biotech.com/documents/download/4.pdf. Accessed ane Mar 2017.
-
Etzkowitz H, Leydesdorff L. The endless transition: a "triple helix" of university-industry-government relations. Minerva. 1998;36(three):203–8.
-
Etzkowitz H, Leydesdorff Fifty. The dynamics of innovation: from National Systems and "Mode 2" to a Triple Helix of academy–manufacture–government relations. Res Policy. 2000;29(2):109–23.
-
Greenhalgh T, Ovseiko PV, Fahy N, Shaw South, Kerr P, Rushforth AD, et al. Maximising value from a United Kingdom Biomedical Research Centre: study protocol. Wellness Res Policy Syst. 2017;fifteen:seventy.
-
Lamine West, Mian S, Fayolle A, Wright M, Klofsten M, Etzkowitz H. Engineering business organization incubation mechanisms and sustainable regional development. J Technol Transf. 2018;43(5):1121–41.
-
Champenois C, Etzkowitz H. From boundary line to boundary space: The creation of hybrid organizations every bit a Triple Helix micro-foundation. Technovation. 2018;76-77:28–39.
-
Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U Southward A. 2005;102(46):16569–72.
-
Pickaert Thou-C. The Value of Innovation. The "Patients W.A.I.T." Indicator 2013. http://www.apifarma.pt/eventos/Documents/Lisbon%20-%20Apifama%20Conference%20-%2013%2006%2028%20w-o%20(2).pdf. Accessed 20 Jun 2018.
-
Schwab K. The Global Competitiveness Report 2010–2011. https://world wide web.weforum.org/reports/global-competitiveness-report-2010-2011. Accessed xx Jun 2018.
-
Owen-Smith J, Powell WW. Noesis networks as channels and conduits: the furnishings of spillovers in the Boston Biotechnology Community. Organ Sci. 2004;15(i):5–21.
-
Harrison C. Patent watch. Nat Rev Drug Discov. 2013;12(ane):14–5.
-
Nadeau P. Venture capital investment selection: do patents attract investors? Strateg Chang. 2010;nineteen(7–8):325–42.
-
Ovseiko PV, Buchan AM. Organizational culture in an academic health center: an exploratory study using a Competing Values Framework. Acad Med. 2012;87(6):709–eighteen.
-
Ovseiko PV, Melham Thou, Fowler J, Buchan AM. Organisational culture and post-merger integration in an academic health centre: a mixed-methods study. BMC Health Serv Res. 2015;15:25.
-
Kuhlmann E, Ovseiko PV, Kurmeyer C, Gutiérrez-Lobos Chiliad, Steinböck South, von Knorring M, et al. Closing the gender leadership gap: a multi-centre cantankerous-country comparing of women in direction and leadership in academic health centres in the European Marriage. Hum Resour Health. 2017;15:2.
-
Ovseiko PV, Greenhalgh T, Adam P, Grant J, Hinrichs-Krapels South, Graham KE, et al. A global telephone call for activeness to include gender in research affect cess. Health Res Policy Syst. 2016;14:fifty.
Acknowledgements
We dedicate this paper to the memory of Dr Alex Casta, our co-author and CEO at UPF Ventures, an innovation management company devoted to boost technology transfer and translational innovation, who tragically passed abroad on iii Dec 2018. Nosotros are grateful to all those who supported and facilitated this research in each of the five regions of the HealthTIES consortium, including those who contributed to the projection during its lifespan, or who were members of the advisory boards and steering committees.
Funding
HealthTIES was funded by an European union FP7 Regions of Knowledge award No. 265550. LDE and PVO are supported by the European union's Horizon 2020 research and innovation programme award STARBIOS2 nether grant agreement No. 709517. ABH, PVO, LDE, ADR and MA are supported by the National Plant for Wellness Research (NIHR) Oxford Biomedical Research Eye, grant BRC-1215-20008 to the Oxford University Hospitals NHS Foundation Trust and the Academy of Oxford. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Availability of information and materials
All data and analyses are available on reasonable request from the corresponding author (ABH).
Author information
Affiliations
Author notes
-
Alex Casta is deceased. This paper is defended to his memory.
- Alex Casta
Contributions
PCWH, EH, ABH, GM and MV conceived and designed the HealthTIES project. LDE, SG, RK, Air conditioning, MVN, MR and ABH participated in data collection and assay. LDE, SG, EH and ABH wrote the first draft of the report from the HealthTIES project. PVO extensively revised the beginning draft with input from ADR and prepared the current manuscript for publication as a research commodity. All authors commented on and approved the final version of the manuscript.
Respective author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
EH is a director of evalueSCIENCE Ltd., PCWH and ABH are advisors. The other authors declare no financial competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Box S1. Search protocol for HealthTIES (HT) H-indexes for disease and technology platforms, Web of Science. Tabular array S1. Keywords used in the HT H-index assay. Figure S1. Radar plots of the HT Innovation index for Input, Innovation System and Output Indicators (with weighted scores) for each region separately: Biocat (The HealthTIES average is shown in grey). Figure S2. Radar plots of the HT Innovation index for Input, Innovation System and Output Indicators (with weighted scores) for each region separately: Észak-Alföld (The HealthTIES average is shown in greyness). Figure S3. Radar plots of the HT Innovation index for Input, Innovation System and Output Indicators (with weighted scores) for each region separately: Medical Delta (The HealthTIES average is shown in grey). Figure S4. Radar plots of the HT Innovation index for Input, Innovation System and Output Indicators (with weighted scores) for each region separately: Oxford and Thames Valley (The HealthTIES average is shown in grey). Figure S5. Radar plots of the HT Innovation index for Input, Innovation System and Output Indicators (with weighted scores) for each region separately: Life Scientific discipline Zurich (The HealthTIES boilerplate is shown in grey). Figure S6. Clustered column diagrams of the HT Innovation index over fourth dimension, 2010 and 2012. Effigy S7. Strengths, weaknesses, opportunities and threats (SWOT) analyses for each region. (PDF 1712 kb)
Boosted file 2:
A business model canvas for bioincubators based on all-time practice in xi bioincubators in Catalonia, South Holland, Zürich, England and Scotland, 2013. (PDF 192 kb)
Rights and permissions
Open Admission This article is distributed nether the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/four.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you requite appropriate credit to the original author(s) and the source, provide a link to the Artistic Eatables license, and indicate if changes were fabricated. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data fabricated available in this article, unless otherwise stated.
Reprints and Permissions
Near this article
Cite this article
Edmunds, L.D., Gluderer, S., Ovseiko, P.V. et al. New indicators and indexes for benchmarking academy–manufacture–government innovation in medical and life science clusters: results from the European FP7 Regions of Knowledge HealthTIES project. Health Res Policy Sys 17, 10 (2019). https://doi.org/10.1186/s12961-019-0414-five
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/ten.1186/s12961-019-0414-5
Keywords
- Regional innovation cluster
- innovation alphabetize
- triple helix
- university–manufacture–government innovation
- Regions of Knowledge
- life sciences
- medical sciences
- biotechnology
- public policy
- European Union
mockridgeexpriver.blogspot.com
Source: https://health-policy-systems.biomedcentral.com/articles/10.1186/s12961-019-0414-5